Fecal Transplants Curing Disease?
Fecal Transplant Therapy
It’s No Joke That Crap Could Save Your Life
Fecal Transplant Therapy or FMT (fecal microbiota transplantation), also known as human probiotic infusion and stool transplant, is a powerful infusion of healthy colonic flora that comes from a healthy donor. Yes, you read that correctly! The person who wishes to receive a fecal transplant receives the feces from a person who has been cleared medically and considered healthy. Why do a fecal transplant? Well, if you can get over the “ik”-factor you might be surprised to learn that this procedure can cure one of the deadliest hospital and community acquired infections around, namely, antibiotic-resistant Clostridium difficile (known as C diff.) infections. FMT can also cure, or dramatically help, inflammatory bowel diseases including Ulcerative Colitis and Crohn’s Disease. Chronic constipation resulting from irritable bowel syndrome can also often responds favorably to FMT. If this procedure is so effective, besides the ik-factor, why aren’t doctors and scientists in favor of its routine use both in the home and hospital setting?
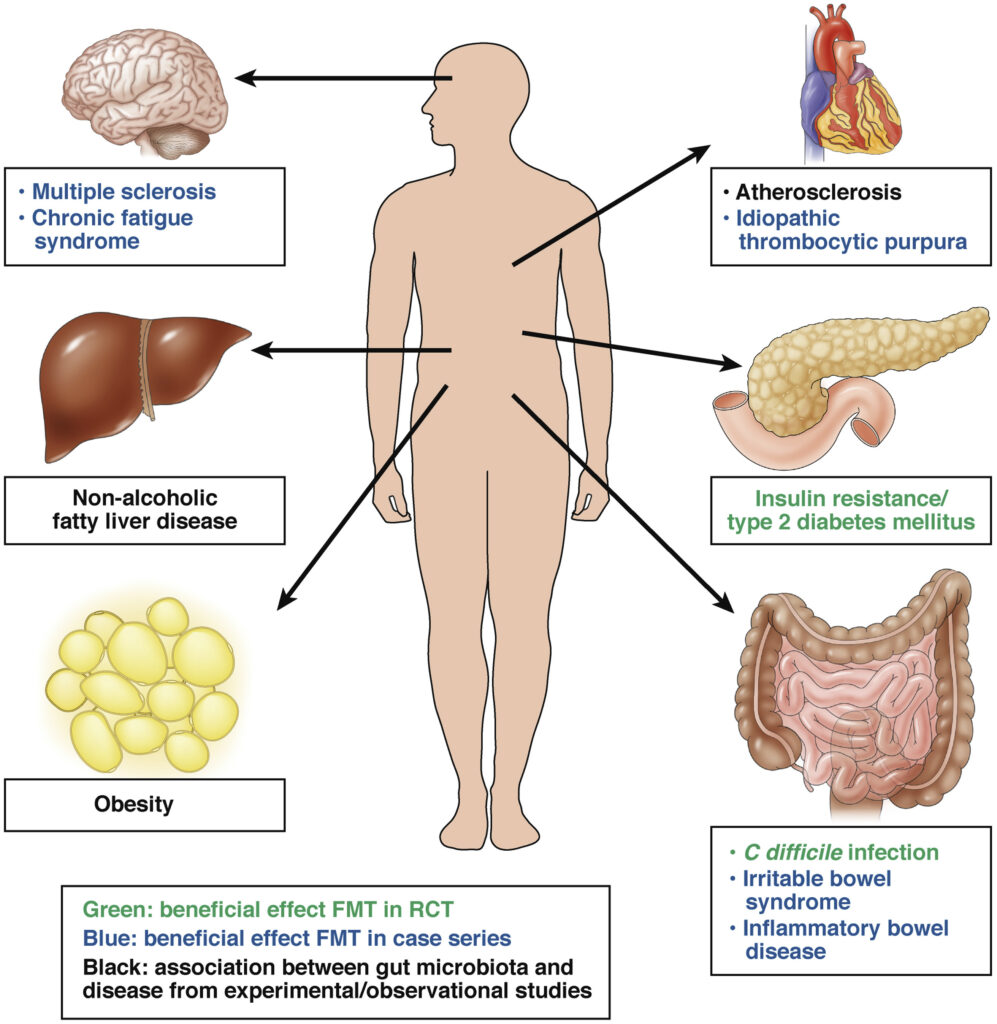
Actually, many medical institutions, and some private office settings, supervise their patients FMT efforts. In the non-hospital or institution setting, complimentary health care practitioners lead the pack in recommending the FMT procedures. From my knowledge, I have been aware of it use, and have guided patient’s through this procedure, no fewer than 10 times. I am also aware that FMT has been used for other medical applications including, multiple sclerosis, lupus, Sjogren’s Syndrome and even colon cancer. Each case that I was personally involved in resulting in zero mishaps or side effects. It seems, from several clinical papers describing FMT use in those with Clostridium difficile that the procedure is very safe and extremely effective.
How Is The Procedure Performed?
There are several variations involving fecal transplant. The use of capsules filled with donor stool can be swallowed or fecal matter can be delivered directly by a gastroenterologist during either an endoscopy procedure (through the mouth) or rectally during a procedure similar to a colonoscopy. The most common method of fecal delivery to the intestinal tract by the lay person is by filling an enema container with fecal matter and inserting and injecting it into the rectum. The rectal fecal transplant procedure has several variations one of which is outlined below. It should be noted that these various methods of fecal transplantation seem to be equally effective although controlled studies have not been carried out up to this point.
ENEMA PROCEDURE
- Collect 50 mL of donor stool in an appropriate sterile container.
- Place immediately into the blender with between 100 – 400mls of normal saline (the volume of saline needed to make mixture ‘pourable’).
- Blend for 15 seconds.
- Ensure the white ball in the enema bag is removed and the white clip is closed on the tubing. Pour the blended mixture into the enema bag via the red cap. Eliminate as much air as possible and close the red cap.
- Once preparation is complete, recipient will lie on their LEFT side in the fetal position with lower half of body elevated.
- Lubricate the rectal tip and gently insert the tip into the anus until you reach halfway of the blue tip. Slowly unclamp the enema bag after hanging the bag up which will to commence the infusion. Allow 5-10 minutes for infusion by the force of gravity.
- Once infusion has been completed, clamp the tubing and gently remove the rectal tip (still attached to the tubing and bag). Discard the enema bag and tip and ‘double bag’ for disposal.
- You then remain on your left side, massaging your abdomen for approximately 10 minutes. Repeat this massage, lying on back, stomach and completing on your right side.
- This procedure is repeated each day for approximately 5 – 10 days; depending upon patient response.
10. If you have difficulty retaining the enema you can take Imodium or codeine as required and approved by your doctor.
The FDA In The Way?
Actually, doctors and researchers are in favor of the use of FMT and recognize its proven ability to save lives, reduce medical costs and avoid less effective therapies such as metronidazole. The reference articles cited herein are just a few of many that prove fecal transplants work for Clostridium difficile and inflammatory bowel disease. The Food and Drug Administration believes that the FMT procedure should be tightly regulated. As reported by a number of news outlets in June 2013, the FDA is attempting to require doctors to complete and submit an “investigational new drug application” (IND) before providing FMT to their critically ill patients. Submittal of the application is not a guarantee of acceptance and could take up to 30 days. However, preparation of this paperwork by the physician itself can take weeks to months! This means that individual physicians have to jump through lengthy paperwork in their attempt to save their patients lives’ with FMT. Consider that the annual death rate from Clostridium difficile is approximately 14,000 with 6.9 percent of those infected dying by the 30th day post diagnosis and nearly 17% by the first year.
One the other hand, it seems reasonable to require some oversight for medical procedures, its important to note that FMT uses a non-prescription substance, namely stool, as part of a procedure that is known to be effective. The commonly used enema method used by lay-people is easy and very safe. Until now, the FDA only required INDs for clinical trials, not for individual patient use. The general public uses millions of enemas each year with very few mishaps. Below is the author’s personal opinion regarding the FDA’s attempts to require tight regulation of this lifesaving procedure.
Dr. Wald’s Opinion On the FDA’s Attempt To Regulate FMT
“I think that the FDA’s position on regulation of fecal transplants by requiring an investigational drug application is overzealous. Fecal matter, to begin with, is not a drug so it makes little sense for the FDA to require an IND. The FDA’s decision will likely cost people their lives as those with Clostridium difficile can succumb to this infection quickly as stated in this paper. C. Difficile infection is deadly and requires urgent medical attention. The waiting period for “potential approval” of the FDA application can take 30 days or more and there is no guarantee of its approval; potentially risking more lives. The fecal transplant procedure is well-proven to be much more effective for curing C. Difficile infection than antibiotics. Inflammatory bowel diseases like ulcerative colitis and Crohn’s Disease also have been show to dramatically benefit from the transplant, or may even be cured. Requiring a doctor to apply for an investigational new drug application, I believe, is yet another attempt by the FDA to stick their noses where they do not belong…namely in the toilet!” A voluntary certification course that establishes a level of standardization is reasonable, but delaying such a simple, safe and effective procedure is simply unnecessary.
I predict that with the new regulation requiring an IND by the FDA will cause physicians to under-utilize FMT to avoid delaying patient care, bypass paperwork and risk of denial of the IND application. Patient’s in need of FMT out of the hospital setting will simply attempt the procedure on their own without medical supervision.”
Reinventing Poop? It has been done!
There is at least one biotechnology company that has come up with artificial poop. That’s right! Synthetic poop made in a laboratory for the purpose of stool transplant instead of real fecal matter for the fecal transplant. The only reason to produce synthetic fecal matter in a lab is because there must be a huge financial incentive to do so. Doctors in Ontario Canada developed the synthetic poop; they call it RePOOPulate. With synthetic stool, there may be the benefits of no longer needing a stool transplant donor, nor to perform screening tests, for the purpose of medical clearance of the donated stool. However, the risks using real stool versus synthetic stool do not seem to justify the costs of using fake poop. Additionally, it is unlikely that a laboratory could replicate the hundreds if not thousands of immune modulators (i.e, endogenous probiotics and cellular messengers) found in the poop of a healthy human being.
Risks of the FMT Procedure
Potential adverse reactions associated with this procedure may include, but are not limited to, internal bleeding, rectal and intestinal irritation and/or inflammation, intestinal rupture, internal bleeding, infections, colonic tears, arrhythmia such as bradycardia from stimulation of the vagus nerve, inflamed and/or ruptured appendix and bowel perforation and death.
Although these risks are serious, FMT therapy has been proven to be extremely safe, rarely resulting in these health risks.
Medical Work-Up Prior To Fecal Transplantation
Before a fecal transplant procedure is performed under medical supervision, a variety of tests are recommended to help ensure that the donated stool does not carry disease that could infect the recipient. Below is a summary of tests for both the donor and recipient involved in the FMT procedure.
Donor Tests
- HIV; human T-cell lymphotropic virus I and II; syphilis; hepatitis A, B, and C; and Helicobacter pylori antibody. Culture and sensitivity, ova and parasites, cryptosporidia, microspora, and CD toxin.
- Prepare fecal specimen within 6 hours of administration to the recipient; preserve at -78 degrees Celsius and re-warm and deliver via enema to recipient.
Recipient Tests
- Routine blood tests as well as tests for serum protein electrophoresis, serum immunoglobulins, HIV, and antigliadin antibodies. Culture and sensitivity, ova and parasites, cryptosporidia, microspora, and CD toxin.
- Start on maintenance therapy with oral S. boulardii (Blood Detective Nutritionals, Saccromyces Boulardii; 500 mg twice daily), plus metronidazole until 24 to 48 hours prior to the transplant.
- Start on maintenance therapy with oral Re-Colonize (a powered supplement containing 220 billion mixed synergistic probiotic organisms per packet) at 2 packets per day (available at: www.blooddetective.com) along with Saccromyces boulardii (a beneficial yeast organism proven by itself to help eradicate C. Difficile and help inflammatory bowel disease) 24 to 48 hours prior to the transplant.
- Drink at least 1 liter of water per day for 3 or more days prior to the procedure.
- Take 2 Immodium tablets on the first morning ONLY of the procedure.
In summary, the FMT procedure has been proven to be very safe and extremely effective. Physicians familiar with the procedure recognize that FMT saves lives. Interruption in the availability and/or procedure in a medical setting will likely result in loss of lives because of delayed implementation of FMT or the standard of care. Reduced usage of antibiotics for those with C. Diff and inflammatory bowel disease should be motivation enough for use of FMT considering that C. Difficile is caused by antibiotic use and the inflammatory bowel diseases, treated with antibiotics ongoingly, can result in antibiotic resistance and predispose to more C. Difficile infection. Political and financial motivations should never come between a person’s life and a needed, proven, effective, life-saving therapy.
References
- JW Gastroenterol Apr 29 2003.
- Silverman MS et al. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010 May; 8:471.
- J Clin Gastroenterol. 2012 Feb;46(2):145-9.
- Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results.
- Kelly CR, de Leon L, Jasutkar N.Women and Infant’s Hospital, Brown University Alpert School of Medicine, Providence, RI, USA, colleen_r_kelly@brown.edu